Adsorption of chiral molecules in oxide-supported heterogeneous catalysts: a model approach

Axe 2 - Matériaux multifonctionnels et environnement
Thèse d'Elisa Meriggio
Recherche menée depuis le 1er octobre 2016
Soutenance de thèse le 24 septembre 2019 à 9.30
Amphithéâtre Charpak - Tour 22-23, Rez-de-Chaussée
Sorbonne Université
4 place Jussieu 75005 Paris
Laboratoires co-porteurs
- Laboratoire de Réactivité de Surface (LRS)
Porteur de projet : Xavier Carrier
Co-encadrant : Vincent Humblot - Institut des Nanosciences de Paris (INSP)
Co-encadrant : Gregory Cabailh
Présentation du projet
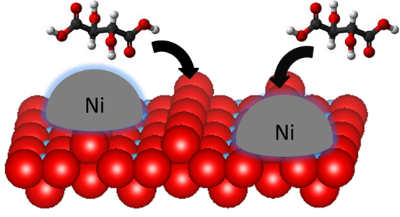
Enantioselective heterogeneous catalysis is a method of choice for the synthesis of enantiopure chiral products. One current approach involves the modification of a metal surface by a chiral modifier. Despite its great potential, only a small number of successful systems have been developed so far. Most of fundamental works have been devoted to model systems based on single crystal metal surfaces while the role of the oxide support in supported metal catalysts have usually been overlooked. To date, fundamental questions remain on the role of the oxide support on the chiral induction. A rational design of the catalyst requires therefore a molecular scale description of the interactions between the oxide support, the metal nanoparticles and the chiral modifier.
In this context, this study aims at understanding the interactions between these three partners through a surface science approach. To mimic the catalytic system, rutile TiO2(110) single crystals, Tartaric Acid molecules (TA) and Ni nanoparticles have been selected. The chemical nature of TA is explored on TiO2(110) by X-ray and Ultraviolet Photoemission Spectroscopy (XPS/UPS) and High Resolution Electron Energy Loss Spectroscopy. Scanning Tunnelling Microscopy (STM) and Low-Energy Electron Diffraction are employed to study the TA layer structure and anchoring points. The molecular decomposition behaviour is studied by Thermal Programmed Desorption (TPD). In parallel, XPS, STM and Surface Differential Reflectivity Spectroscopy are used to probe the growth of Ni NPs on TiO2 at increasing Ni coverage. Finally, perspectives on the TA/Ni/TiO2 system are put forward mainly by XPS and TPD.
Communications orales
- 2019, IUPAC, International Union of Pure and Applied Chemistry, Paris (France)
Model oxide-supported enantioselective catalysts: chemical nature of tartaric acid on rutile TiO2(110) - 2019, JSE, Journées des Spectroscopies d’Électrons, Nancy (France)
Vers un catalyseur énantiosélectif supporté sur oxyde : étude de la nature chimique de l’acide tartrique sur le TiO2 rutile (110) - 2018 JED397, Journées de l’École Doctorale 397, Paris (France)
Understanding chirality in oxidesupported heterogeneous catalysts: a model approach - 2018, Journée scientifique AXE 2 Labex Matisse, Paris (France)
Understanding chirality inoxide-supported heterogeneous catalysts: a model approach - 2018, Elspec’2018, 8ème conférence sur les spectroscopies d’électrons, Biarritz (France)
Catalyseur énantiosélectif supporté sur oxyde : étude de la nature chimique de l’acide tartrique sur le TiO2 rutile (110) par XPS et HREELS - 2017, ECOSS-33, 33rd European Conference on Surface Science, Szeged (Hungary)
Model TiO2-supported enantioselective catalysts
Communications posters
- 2018, JED397, Journées de l’École Doctorale 397, Paris (France)
Understanding chirality in oxidesupported heterogeneous catalysts: a model approach - 2017, Journée du Labex Matisse, Paris (France)
Understanding chirality in oxide-supported heterogeneous catalysts: a model approach - 2017, JSE, Journées Scientifiques du Comité Spectroscopies d’Électrons, Paris (France)
Identification of the chemical nature of tartaric acid on rutile TiO2(110) by XPS: a comparative study with Au(111) and Cu(110)
Liste des publications
- Adsorption of a chiral modifier on an oxide surface: chemical nature of tartaric acid on rutile TiO2(110)
E. Meriggio, R. Lazzari, C. Méthivier, P. David, S. Chenot, X. Carrier, G. Cabailh, V. Humblot
Appl. Surf. Sci., 2019, 493, 1134-1141 - Chemical nature and thermal decomposition behavior of tartaric acid multilayers on rutile TiO2(110)
E. Meriggio, R. Lazzari, C. Méthivier, P. David, S. Chenot, X. Carrier, G. Cabailh, V. Humblot,
J. Vac. Sci. Technol. B, 2019, 37(5):051803 - Transport in ITO Nanocrystals with Short- to Long-Wave Infrared Absorption for Heavy-Metal-Free Infrared Photodetection
J. Qu, C. Livache, B. Martinez, C. Gréboval, A. Chu, E. Meriggio, J. Ramade, H. Cruguel, X. Z. Xu, A. Proust, F. Volatron, G. Cabailh, N. Goubet, E. Lhuillier
ACS Appl. Nano Mater., 2019, 2, 1621-1630 - Polyoxometalate as Control Agent for the Doping in HgSe SelfDoped Nanocrystals
B. Martinez, C. Livache, E. Meriggio, X. Z. Xu, H. Cruguel, E. Lacaze, A. Proust, S. Ithurria, M. G. Silly, G. Cabailh, F. Volatron, E. Lhuillier
J. Phys. Chem. C, 2018, 122, 26680-26685 - Photoemission Fingerprints for Structural Identification of Titanium Dioxide Surfaces
P. Borghetti, E. Meriggio, G. Rousse, G. Cabailh, R. Lazzari, J. Jupille,
J. Phys. Chem. Lett., 2016, 7, 3223-3228
Egalement dans la rubrique
- Thèse de Jonathan Baptista
- Thèse de Florian Beaugnon
- Thèse d'Emmanuelle de Clermont Gallerande
- Thèse d'Elise Duquesne
- Thèse de Cyrine Ernandes
- Thèse d'Ali Fakih
- Thèse d'Amaury Fau
- Thèse de Camille Lagoin
- Thèse de Miléna Lama
- Thèse de Danilo Longo
- Thèse de Lorela Masci
- Thèse de Bertille Martinez
- Thèse d'Elise Médina
- Thèse d'Estelle Palierse
- Thèse de Karol Rakotozandriny
- Thèse de Sofiane Schaack
- Thèse de Lionel Tinat
- Thèse de Hancheng Yang
- Thèse de Dan Zhao
MATISSE en chiffres
- 4 disciplines : Chimie, Physique, Sciences de la Terre, Patrimoine
- 400 permanents
Contact
Direction
Florence Babonneau
Administration
Communication
Emmanuel Sautjeau



