L’eau adsorbée, site actif pour la catalyse ? Cas de la réaction de transestérification sur argiles
Axe 3 - Interfaces, transport et réactivité
Post-doctorat de Elisa Silva Gomes
Projet de recherche commencé le 1er octobre 2017.
Laboratoires co-porteurs
- Laboratoire de Réactivité de Surface (LRS)
Porteur de projet : Hélène Lauron-Pernot
Co-encadrants : Guillaume Laugel - Laboratoire d'Archéologie Moléculaire et Structurale (LAMS)
Co-encadrants : Maguy Jaber - Laboratoire PHENIX
Co-encadrants : Laurent Michot
Mots clés
transestérification, argiles, catalyse
Projet de recherche
The development of clean processes for the recovery of biomass molecules is a necessity, whether to produce fuels or raw materials for the chemical industry. This objective strongly orientates research in the field of heterogeneous catalysis towards the implementation of liquid phase processes, either in aqueous medium or without solvent, but in the presence of residual water adsorbed on the catalyst or in the reactive mixtures. The role of water on the reaction is then a key parameter governing reactivity. It is the case in the transesterification reaction in liquid phase that is important for the production of biodiesel from vegetable oils but also for the opening of lactones accessible from the biomass and allowing access to monomers for polymerization. The study of this type of reaction carried out in LRS showed the very good catalytic activity magnesium silicates containing a magnesium silicate hydrate (MSH) phase, which has similar structure to a defectuous talc, (a TOT clay); and it was also shown that molecular chemisorbed water on accessible Mg2+ cations enhanced the activity. Motivated by this study, the aim of this project is to study the effects of water structuring in magnesium clay minerals on the catalytic activity of transesterification in liquid phase. The approach will consist in varying some key parameters known to have an influence on water structuration i.e. particle size, layer charge, and nature of the interlayer cation of selected clay minerals, test the catalytic activity of the resulting materials and investigate the physicochemical properties of the most representative materials.
Scientific results
To investigate the catalytic activity of the clay minerals, we chose the simple reaction of transesterification of ethyl acetate (AcOEt) by methanol (MeOH) in liquid phase.
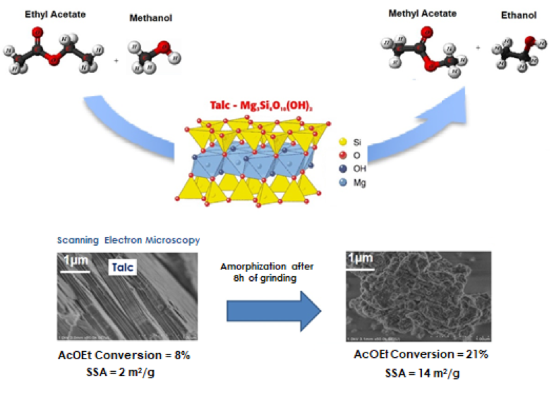
As a magnesium silicate sample containing a MSH phase (very similar to a defectuous talc) showed in preliminary studies a very interesting activity, we tested a natural talc. Then, the influence of particle size was studied by evaluating the activity of nanotalc that possess the same structure as talc, but with shorter length of particles. It’s worthy to mention that talc is a layered material, and each layer is composed by a Mg octahedral sheet sandwiched between two silicon tetrahedral sheets. It presents hydroxyl species at the edges of the sheets and also at the octahedral sheet, which can be exposed due to the occurrence of structural defects. Those species can act as catalytic sites.
The magnesium silicate exhibited the highest value of specific surface area (SSA = 421 m2/g) and 29 % of AcOEt conversion. Despite the possible structural similarities, talc exhibited lower AcOEt conversion (8%), but also lower SSA (2 m2/g) when compared to the magnesium silicate. For nanotalc, 57% of AcOEt conversion and 130 m2/g of SSA were observed.
Once nanotalc exhibited higher activity than talc, we carried out the grinding of talc for different periods of time (from 30 min. to 8 h) in order to study if the improved performance of nanotalc was due to a surface effect or to the creation of active sites. When tested on transesterification, the grinded samples showed an increase of AcOEt conversion with the increase of grinding time. But there was no direct correlation between the increase of SSA and the increase of AcOEt conversion on the grinded samples: the sample with higher value of SSA (grinded for 1h) did not show the highest activity, showing that the improved activity of talc is not due to a mechanical surface effect. The characterization of the talc samples before and after grinding by X-ray diffraction and Scanning electron microscopy allowed us to observe that before grinding, the talc exhibited high crystallinity and well defined layers, and after 8h of grinding the material became amorphous, with the presence of structural defects. The hydrophilic/hydrophobic properties of talc samples were evaluated by mixing the sample with cyclohexane and water. It was observed that the talc after 8h of grinding exhibited improved hydrophilicity that can be due to the formation of structural defects (like the MSH phase), allowing the exposure of the hydroxyl groups present at the octahedral sheets, leading to high activity of this sample in transesterification reaction. So one can conclude that, on talc-like structures, the transesterification of small chain esters is favored on the edges and structural defects.
In parallel, TOT clays with negatively charged sheets are prepared to study the influence of the exchanged cations on water structuration. The work is focused on laponite type materials that exhibit a higher activity than the commercial magnesium silicate. The structuration of the clay aggregates in the liquid phase is also investigated as it has been identified as a key parameter.
Egalement dans la rubrique
- Co-adsorption de l’eau et de peptides sur des surfaces métalliques
- Dégradation oxydative des contaminants à la surface de nanoparticules contenant du Fe(II) : nouveaux mécanismes sans oxydant fort
- Étude par dynamique moléculaire ab% initio de la photo=électrolyse de! l’eau sur des surfaces d’anatase fluorée
- Investigation of electrochemical and electromechanical properties of nanostructured LiCoO2 electrodes for energy storage devices
- MRI study of alumina support impregnation by aqueous, metal salt solutions
- Recyclage du carbone en zone de subduction : approche naturaliste
MATISSE en chiffres
- 4 disciplines : Chimie, Physique, Sciences de la Terre, Patrimoine
- 400 permanents
Contact
Direction
Florence Babonneau
Administration
Communication
Emmanuel Sautjeau